Welcome to Matrix Education
To ensure we are showing you the most relevant content, please select your location below.
Select a year to see courses
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Learn online or on-campus during the term or school holidays
Get HSC exam ready in just a week
Select a year to see available courses
Science guides to help you get ahead
Science guides to help you get ahead
Do you think you're ready for Year 9 Chemistry? This guide will show you! Learn about atoms, elements, and the periodic table to get ahead.
Join 75,893 students who already have a head start.
"*" indicates required fields
Related courses
When you studied Science in Year 7 and Year 8 you would have learnt about the many tiny particles that make up our world. You learnt that atoms come in different elements, and that those elements could combine together to make compounds. But in Year 9 Chemistry topics, you need to go deeper… deeper into the atom!
Your first step into Year 9 Chemistry—test your skills with these practice questions Fill out your details below to get this resource emailed to you. "*" indicates required fields
Get ahead in Year 9 Science!

Get ahead in Year 9 Science!
Table of contents:
This article is part of our Year 9 Science Syllabus Series. Check out the other articles in the series: Year 9 Biology Syllabus and Year 9 Physics Syllabus.
The original model of the atom, created by Thomas Dalton in the 1800s, was a smooth, featureless sphere. It was defined as the smallest particle that could ever exist. It was named “atom” because it means “indivisible” in Greek.
However, by the early 20th Century, the first subatomic particle was observed by J.J. Thomson. Atoms were divisible after all!
New discoveries came quickly after that, and scientists such as Ernest Rutherfod, Niels Bohr and Erwin Shrodinger all proposed updated models for the atom.
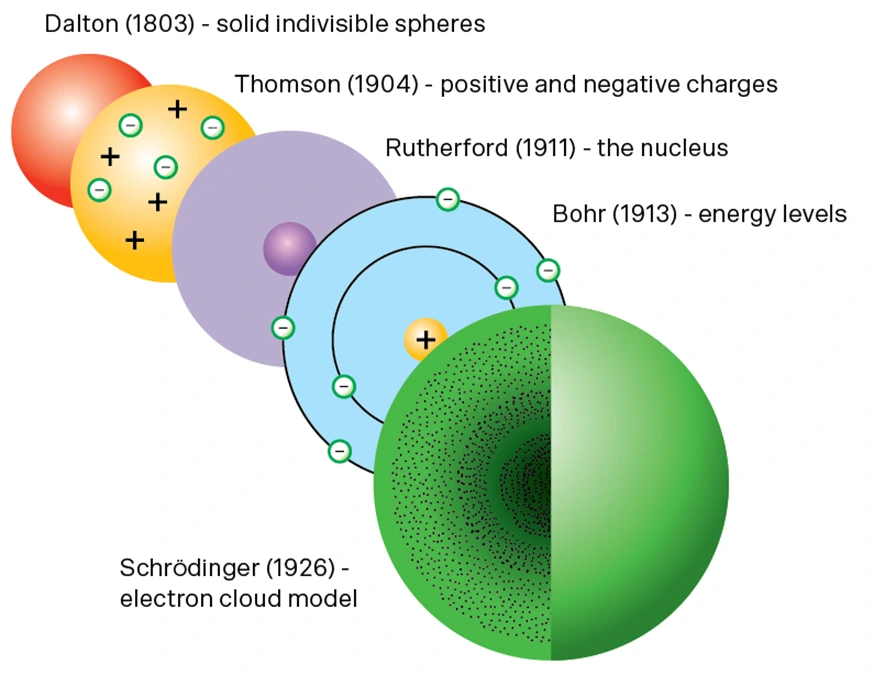
So, are you ready to:
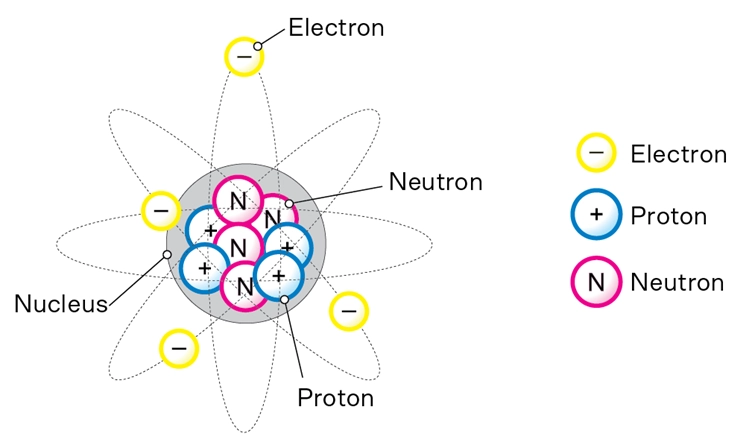
We’ll be using the Bohr model to solve problems in Year 9 Chemistry topics. According to the Bohr model:
The rest of the atom (most of it, actually) is empty space.
The way the subatomic parts of atoms are arranged explains how they join together to form all the materials we see every day. This basic structure is a key idea in Year 9 Chemistry topics and in the world of Science.
So, are you ready to:
Before scientists knew about atoms, we gained indirect knowledge by studying the different elements. For example, we discovered that each element had a different mass.
Scientists also noticed that certain elements had similar chemical properties.
Many scientists tried to group the elements into families or trends but had limited success. They were working with limited knowledge – some of the elements they would need to complete the pattern hadn’t even been discovered yet!
Dmitri Mendeleev conducted the most in-depth and organised investigation of the properties of elements in the 19th century.
This led to the invention of the periodic table – a way to organise elements by their number of subatomic particles, created before we even knew subatomic particles existed!
Fun fact: Mendeleev was so sure that his organisation of the elements was correct that he left gaps where elements should exist but didn’t, and later those elements were discovered!
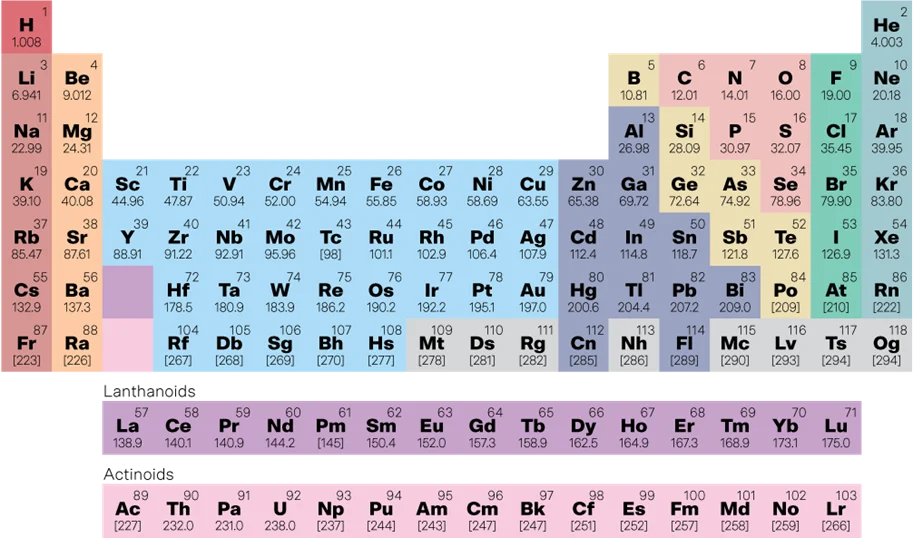
The periodic table includes:
Periods (rows): 7 in total
Groups (columns): 18 in total
Regions: Metals, non-metals, metalloids
Special blocks like the transition metals (Groups 3-12), lanthanides (Elements 57-71), and actinides (elements 93-103).
Periodic table tips to remember:
So, are you ready to:

Write perfect science reports with this expert-made template!

Fill out your details below to get this resource emailed to you.
"*" indicates required fields
Every element has a unique atomic number, which equals the number of protons in the nucleus. For example:
Hydrogen has 1 proton.
Helium has 2.
Oganesson, the heaviest known element, has 118 – this had to be created in a lab because it doesn’t exist in nature..
When you look up an element on the periodic table, you find that its atomic number tells you how many protons it has.
The atomic mass is the total number of protons and neutrons in the nucleus. Electrons are so light that they don’t contribute to the mass. It is measured in atomic mass units, where neutrons and protons both have a mass of 1 and electrons have a mass of zero.
Neutrons help bind the nucleus together. A nucleus containing only positive protons would fall apart because of the protons repelling each other.
This is an important concept to know in Year 9 Chemistry topics.
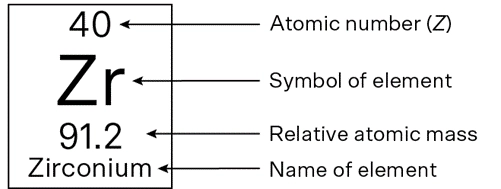
You can find the average atomic mass (also called the relative atomic mass, or relative atomic weight) by looking on the periodic table below the element’s symbol.
But here’s a twist: elements can exist in different versions called isotopes—atoms with the same number of protons but different numbers of neutrons.
This is why many atomic masses on the periodic table are decimals; they are averages of all isotopes found in nature.
For example, neon-20 has 10 protons and 10 neutrons and weighs 20 exactly, but about 9% of neon in the world is neon-22, which has 10 protons and 12 neutrons. This gives neon an average mass of 20.2.
So, are you ready to:
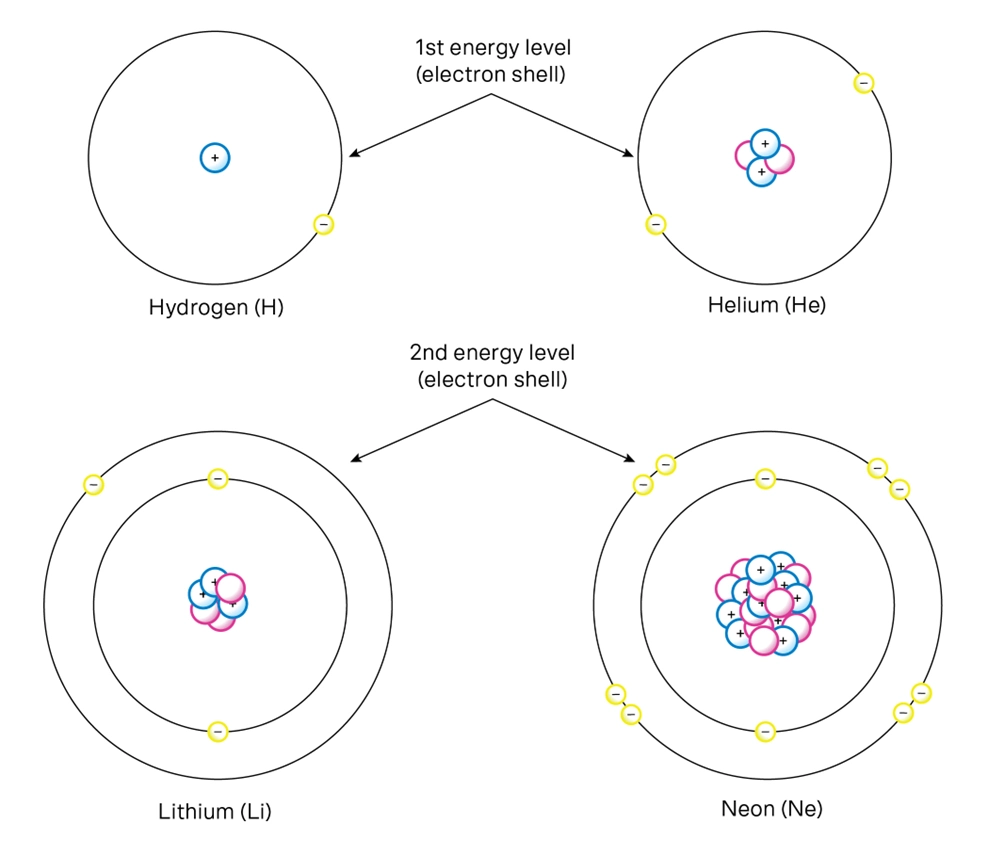
The number of electrons in an atom will equal the number of protons.
Electrons are arranged in shells, or energy levels, around the nucleus. These fill in a predictable pattern:
The 1st shell holds 2 electrons.
The 2nd and 3rd shells hold up to 8 each.
This matches the structure of the periodic table. The first row of the periodic table has two elements. The next two shells can fit eight electrons each, which is why the next two rows contain eight elements. After element 20 (Calcium), things get more complex, but for now, you’ll focus on simpler atoms.
The most important shell for Year 9 Chemistry topics is the valence shell—the outermost shell. The number of electrons in this shell determines an element’s chemical properties. For example, Group 1 elements like sodium and lithium all have 1 valence electron and react explosively with water – this is why they’re Group 1.
So, are you ready to:
Studying Year 9 Chemistry topics requires you to train your memory, use logic to solve problems, and understand how scientists use models to make sense of the world. The concepts you learn this year are crucial building blocks for your future studies.
In Year 10, you’ll use this knowledge to predict chemical reactions.
In Year 11, you’ll explore how electron arrangements affect materials.
In Year 12, you’ll learn how scientists apply these properties to design batteries, make medicines, and tackle environmental problems.
And it all starts here, in Year 9, with understanding the atom!
Your first step into Year 9 Chemistry—test your skills with these practice questions Fill out your details below to get this resource emailed to you. "*" indicates required fields
Get ahead in Year 9 Science!

Get ahead in Year 9 Science!
Written by Matrix Science Team
The Matrix Science Team are teachers and tutors with a passion for Science and a dedication to seeing Matrix Students achieving their academic goals.© Matrix Education and www.matrix.edu.au, 2023. Unauthorised use and/or duplication of this material without express and written permission from this site’s author and/or owner is strictly prohibited. Excerpts and links may be used, provided that full and clear credit is given to Matrix Education and www.matrix.edu.au with appropriate and specific direction to the original content.